Description
Assess the metabolic liability and potential of novel active ingredients (chemicals, plant extracts, biopesticides, etc) to break insecticide resistance
Material provided: Summary report on the metabolic liability and efficiency of novel compounds against highly resistant lines
Unit definition: A complete screen against >5 detoxification enzymes and >5 drosophila/insect strains
Novel active ingredients (new chemicals, re-purposed chemicals, plant extracts, microbial metabolities, biopesticides, etc) that could serve as environmentally-friendly selective insecticides for vector control, with low toxicity to humans and non-target organisms are currently being developed. The ability of these “leads” to tackle insecticide resistance is a critical parameter, to determine their actual potential for further development.
This service is offering in vitro and in vivo evaluation assays for screening putative novel active ingredients, to predict their metabolic liability and potential to break insecticide resistance.
The service includes:
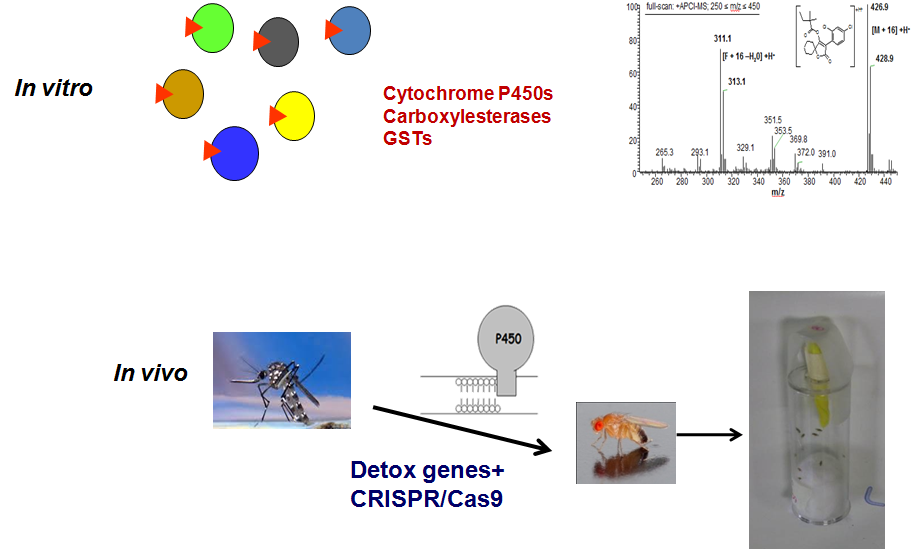
(a) In vitro assays using major detoxification enzymes, from the most resistant insect pests and HPLC/GCMS analytical systems
(b) In vivo assays, using highly resistant genetically defined Drosophila/insect lines with combinations of target site mutations and detoxification enzymes.
Description of the experiment and the product
The user should provide compounds, with pesticide properties, at the appropriate amounts and forms (relevant to its bioactivity range). Consultation for the sampling procedures can be provided by the Provider.
We will handle and analyze the samples provided by the user with the following workflow:
In vitro screens:
Step 1: Assess the metabolic liability through in vitro assays: incubation with available recombinant detoxification enzymes – major pesticide metabolisers, alongside control insecticides and model substrates.
Step 2: Analysis of parental compound and/or its metabolites by HPLC/LCMS.
In vivo screens:
Step 1: Preparation of appropriate formulations, using the “compound” provided in larvae medium or contact assay device.
Step 2: Use of highly resistant drosophila/insect lines with defined insecticide resistance mechanisms, to determine compound efficiency, compared to control lines with no resistance mechanisms.
Data layout
The data that will be returned to the user will consist of both raw data (bioassay results and outcomes of analytical approaches – chromatograms etc), as well as summary report, for the metabolic liability and efficiency of the compounds against highly resistant lines. Additionally, reports of the procedure and data interpretation will be also made available upon request.
Publications
- Douris V. et al Resistance mutation conserved between insects and mites unravels the benzoylurea insecticide mode of action on chitin biosynthesis Proc Nat Acad Sci USA (PNAS) 113 p.14692–14697
- Grigoraki L. et al (2016) Functional and immunohistochemical characterization of CCEae3a, a carboxylesterase associated with temephos resistance in the major arbovirus vectors Aedes aegypti and Ae. albopictus. Insect Biochem Mol Biol 74, p. 61-67
- Riga M. et al (2015) Functional characterization of the Tetranychus urticae CYP392A11, a cytochrome P450 that hydroxylates cyenopyrafen and fenpyroximate, Insect Biochem Mol Biol 65, 91-99
- Pavlidi N. et al. (2012) Transgenic expression of the Aedes aegypti CYP9J28 confers pyrethroid resistance in Drosophila melanogaster. Pest Bioch Physiol 104 p.132-135.
- Stevenson BJ, Pignatelli P, Nikou D, Paine MJ. (2012) Pinpointing P450s associated with pyrethroid metabolism in the dengue vector, Aedes aegypti: developing new tools to combat insecticide resistance. PLoS Negl Trop Dis. 2012;6:e1595.
- Müller P1, Warr E, Stevenson BJ, Pignatelli PM, Morgan JC, Steven A, Yawson AE, Mitchell SN, Ranson H, Hemingway J , Paine MJ, Donnelly MJ. (2008) Field-caught permethrin-resistant Anopheles gambiae overexpress CYP6P3, a P450 that metabolises pyrethroids. PLoS Genet. 2008 Nov;4(11):e1000286
For more information, please contact us.